Abstract
Background: Iron refractory iron deficiency anemia (IRIDA) is a rare autosomal recessive disorder, caused by a genetic defect in TMPRSS6. This results in increased signaling through the bone morphogenic protein (BMP)/sons of mothers against decapentaplegic (SMAD) pathway, leading to disproportionally high levels of hepcidin relative to body iron status. Hepcidin reduces iron transport to the plasma by degrading the cellular iron exporter ferroportin, resulting in systemic iron deficiency and microcytic anemia. Symptomatic interventions such as intravenous iron administration results in iron sequestration in the reticular endothelial system (RES) and generally only a partial response of hemoglobin concentrations. Further, the potential long-term adverse effects of chronic intravenous iron therapy and associated elevated RES-iron levels are unknown in these patients. Blood transfusion can be administered in cases of severe symptomatic anemia but is generally avoided due to transfusion-related risks. Currently, there is an unmet need for more effective, safe, convenient, and direct-acting treatments for IRIDA. Given the central role of aberrant BMP/SMAD signaling in hepcidin production, selective inhibition of the BMP receptor ALK2 to reduce hepcidin represents a rational approach to target a precipitating factor of IRIDA.
Preclinical studies in the TMPRSS6 siRNA knockdown mouse model of IRIDA demonstrated that treatment with a selective ALK2 inhibitor reduced hepcidin, increased serum iron, and ameliorated anemia. KER-047 is a novel, oral, investigational, small-molecule kinase inhibitor with potent and selective inhibitory activity against the ALK2 receptor. In a Phase 1 clinical trial, single-ascending and multiple-ascending doses of KER-047 administered to healthy participants resulted in robust decreases from baseline in hepcidin levels, increases in serum iron and transferrin saturation (TSAT), decreases in serum ferritin, and increases in reticulocyte hemoglobin content (Ret-He). Based on these observations, KER-047 was advanced to Phase 2 clinical trials.
Aims: The primary objective of this ongoing Phase 2 clinical trial is to evaluate the safety and tolerability of ascending doses of KER-047 in participants with IRIDA. Secondary objectives include evaluation of pharmacokinetic (PK) and pharmacodynamic (PD) parameters.
Methods: This is a 2-part open label, dose escalation and dose expansion trial. Part 1 (dose escalation) consists of up to 4 ascending dose cohorts starting with 25 mg q.d. Part 2 will be the dose expansion phase with safety as the primary focus. Patients diagnosed with IRIDA may enroll in all cohorts in Parts 1 and 2.
Key inclusion criteria include diagnosis of IRIDA based on a homozygous or compound heterozygous TMPRSS6 genotype, a TSAT <15%, a ferritin level 50-700 µg/L and hemoglobin <13.8 g/dL (males) or <12.7g/dL (females) and signed informed consent. Patients are excluded if they have received IV iron treatment within 28 days prior to trial entry. Participants will be treated once daily with KER-047 for a 2-week period, followed by a 2-week washout period. Data are analyzed by using descriptive statistics.
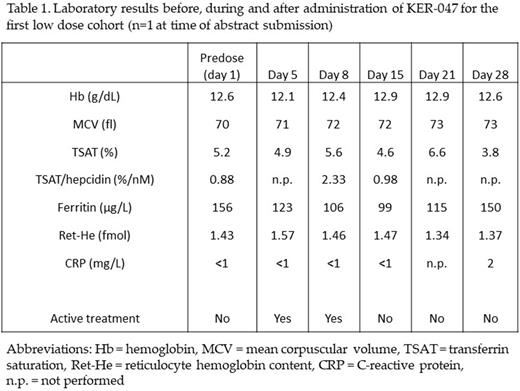
Results: At the time of this abstract submission, one participant had enrolled in the first dose-expansion cohort (KER-047 25mg q.d.). This participant reported intermittent dizziness, however no serious adverse events were observed during treatment. Hemoglobin concentration and mean corpuscular volume remained stable. Ret-He increased by day 5 of treatment. Ferritin levels decreased during treatment and returned to baseline levels after the 2-week treatment period. TSAT/hepcidin ratio, which is characteristically below reference intervals in IRIDA patients, was slightly increased but returned to baseline levels after 2-weeks treatment.
Conclusion: Data are preliminary and limited to a single participant thus far in whom KER-047 25mg q.d. was well tolerated. Changes in red cell and iron parameters at this low dose support development of KER-047 as an agent to potentially address an underlying cause of IRIDA. At the time of this abstract submission, two additional patients are scheduled for screening to complete the first dose cohort. We anticipate data from the total of three patients in time for the presentation to further characterize safety of KER-047 and substantiate effects on iron metabolism.
Disclosures
Hoving:Keros Therapeutics: Research Funding. Donker:Keros Therapeutics: Research Funding. Vainorius:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company; United Therapeutics: Ended employment in the past 24 months. Natarajan:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Lachey:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Cooper:Anokion Therapeutics: Current equity holder in private company, Ended employment in the past 24 months; Kadmon Corporation: Ended employment in the past 24 months; Keros Therapeutics: Current Employment. Swinkels:Keros Therapeutics: Research Funding. Schols:Keros Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal